Audible Mouse Project
Audible Mouse Project Title
In Vivo Cardiac MR Elastography on Mouse
Yifei Liu1, Thomas J Royston1,2, and E Douglas Lewandowski3,4
1Department of Mechanical & Industrial Engineering, University of Illinois at Chicago, Chicago, Illinois, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, Illinois, United States, 3Center for Cardiovascular Research, University of Illinois at Chicago, Chicago, Illinois, United States, 4Department of Physiology & Biophysics and Medicine (Cardiology), University of Illinois at Chicago, Chicago, Illinois, United States
Audible Mouse Project Body
Target Audience: Physicians interested in the diagnosis of cardiac malfunction based on myocardium stiffness.
Background and purpose: Increased stiffness of the left ventricle (LV) wall is a contributing factor to the abnormal function associated with both impaired systolic and diastolic function during the progression of cardiomyopathies to overt heart failure 1. The etiologies of impaired contractility in the failing heart have been associated with increased cardiac fibrosis, steatosis, and altered sarcomere activity and tension, all factors influencing myocardial stiffness 2, 3. The mouse is a common animal model for studying these processes and is readily for the application of MR Elastography, a non-invasive method to estimate stiffness of tissue under harmonic vibration 4. This study demonstrates the feasibility of in vivocardiac MR Elastography (MRE) on the mouse heart to monitor myocardial stiffness, and the stiffness ratio between end-diastole and end-systole.
Methods: In vivocardiac MRE was performed in a 9.4 T Agilent horizontal bore preclinical MR scanner (310/ASR, Santa Clara, CA) on four wild type female mice. The mice were positioned in the supine position, kept under anesthesia with 1.5% isoflurane, in a customized cradle with a nose cone connected to a vaporizer. A 3D printed tube tip was connected to an audio speaker (11829BT, Electro Voice, MN, USA) through a rigid PVC pipe on one end and placed on the shaved left thorax area of the mouse on the other end. MRE was performed on a short-axis slice at the middle level of the left ventricle. Mechanical waves were introduced into the heart by the speaker at 400 Hz, driven by an audio amplifier (P3500S, YAMAHA, Japan).A fractional encoding, ECG gated, cine-MRE sequence was applied for wave image acquisition. The actuation was triggered right after the ECG trigger and turned off after the MEG finished in the last cardiac phase acquisition. The RF pulse for the first cardiac phase acquisition was started after ~20 ms of the actuation trigger in order to give enough time for the acoustic wave to travel from the speaker to the mouse body. MR imaging parameters includes FOV=3×3 cm, acquisition matrix = 128×128, slice thickness = 1mm, flip angle = 20°. TR and TE depend on the heart beating rate and number of cardiac phases acquired. A typical TR/TE = 10.09/ 2.22 ms with 14 phases covers 1.5 cardiac cycles with a heart beating rate of 515 bpm. Three separate MRE scans were performed adding one cycle of 1 kHz, flow compensated shape, MEG with strength of 20 Gauss/cm on the read, phase or slice gradient directions respectively to measure the motion in-plane and through-plane. Eight phase offsets covering one vibration cycle were acquired for each MRE scan. A weighted sum effective stiffness map of the entire body was estimated by a direct inversion algorithm 4 . An ROI of the left ventricle was semi-automatically selected based on the wave amplitude image for each cardiac phase to examine stiffness of the left ventricle.
Audible Mouse Project Body Continued
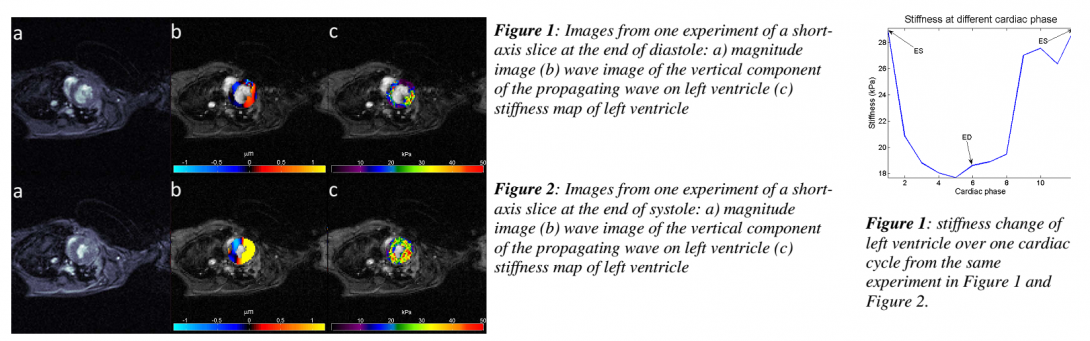
Results: Figure 1 and Figure 2 show the result from one experiment at the end of diastole (ED) and at the end of systole (ES), includingthe magnitude image, wave image of vertical component of motion and stiffness map of the LV overlapping the magnitude image. The mean stiffness overthe ROI is 18.9 kPa at ED phase in Figure 1 (c) and 29 kPa at ES phase in Figure 2 (c). Figure 3 shows the stiffness change in the LV during one cardiac cycle. For the four mice the effective stiffness in end diastole ranged from 7.84 to 18.9 kPa, and 14.07 to 28.9 in end-diastole. And, the stiffness ratio of ED/ES ranged from 0.51 ~ 0.65.
Discussion: Cardiac MRE has been implemented in vivofor human and porcine model studies 5, 6. The present study illustrated the challenges and, ultimately, feasibility of in vivocardiac MRE on the anesthetized, live mouse, for estimating myocardial stiffness in the heart. Further studies will be focusedon the comparison of stiffness ratio difference between normal and murine models of heart disease.
Acknowledge: Financial support of NIH Grant No. HL113057 is acknowledged.
Audible Mouse Project References
References
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005; 7:223-53.
- Conrad CH, Brooks WW, Hayes JA, et al. Myocardial Fibrosis and Stiffness With Hypertrophy and Heart Failure in the Spontaneously Hypertensive Rat. Circulation. 1995; 91:161-70.
- Hankiewicz JH, Banke NH, Farjah M, et al. Early impairment of transmural principal strains in the left ventricular wall after short-term, high-fat feeding of mice predisposed to cardiac steatosis. Circ Cardiovasc Imaging. 2010; 3:710-7
- Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001; 5:237-54.
- Kolipaka A, Aggarwal SR, McGee KP, et al. Magnetic Resonance Elastography as a Method to Estimate Myocardial Contractility. Journal of Magnetic Resonance Imaging. 2012; 36:120-7.
- Sack I, Rump J, Elgeti T, et al. MR Elastography of the Human Heart: Noninvasive Assessment of Myocardial Elasticity Changesby Shear Wave Amplitude Variations. Magnetic Resonance in Medicine. 2009; 61:668-77.